2021 Vol. 6, No. 3
Display Method:
Cover info & Content
2021, 6(3): 309-310.
doi: 10.1016/j.gee.2021.05.005
Abstract:
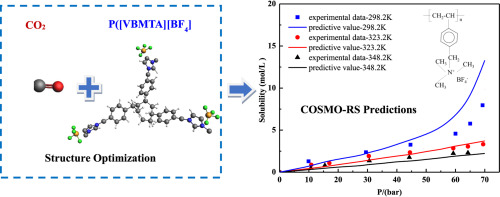
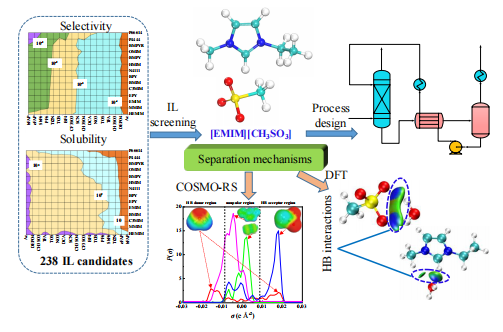
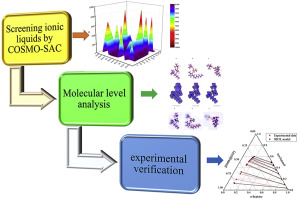
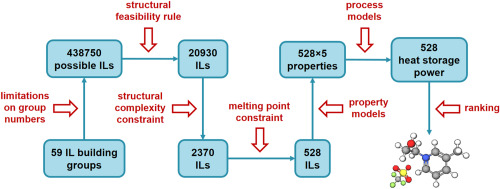
2021, 6(3): 350-362.
doi: 10.1016/j.gee.2020.10.022
Abstract:
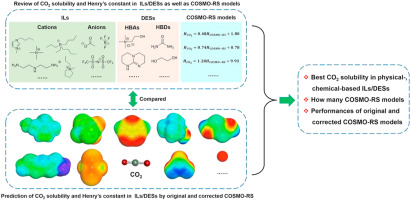
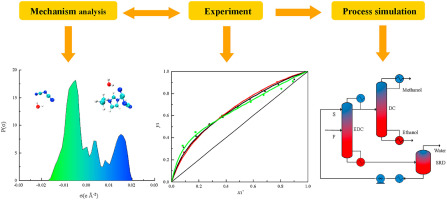
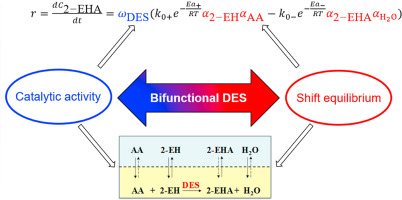
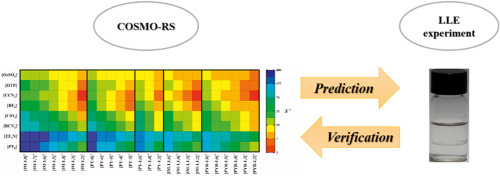
2021, 6(3): 371-379.
doi: 10.1016/j.gee.2020.11.020
Abstract:
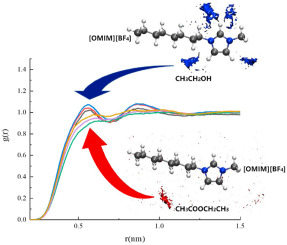
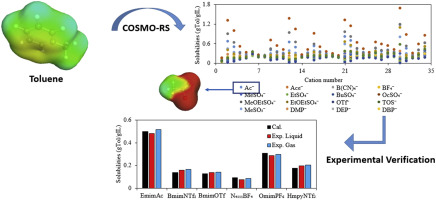
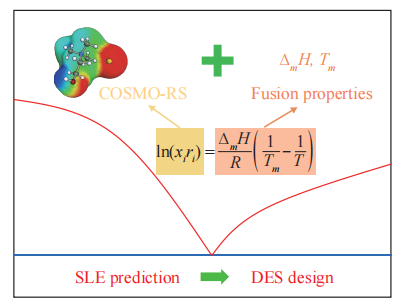
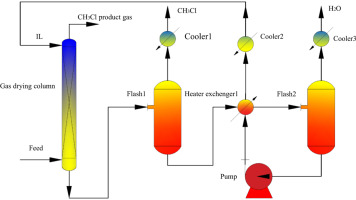
2021, 6(3): 432-443.
doi: 10.1016/j.gee.2020.12.019
Abstract:







 京公网安备 11010802039050号
京公网安备 11010802039050号